Zoonosis
From the Series: Lexicon for an Anthropocene Yet Unseen
From the Series: Lexicon for an Anthropocene Yet Unseen

We are at the cusp of this new age of the Anthropocene, an age in which humans have colonized evolutionary time. To contain and prevent pandemics, scientists are beginning to drive snippets of altered genomes down generations in order to wipe out pernicious insects. The rise of zoonotic diseases, those that spill over from animals to humans directly or (in some definitions) through insect vectors, compels these risky experiments. We are adapting to zoonotic conditions less by taking steps to reduce our heavy footprint on the planet, and more by meddling with nature on a deeper level.
Pathogens have always circulated among animal reservoirs, vectors, and human hosts. Gilles Deleuze and Félix Guattari (1987, 11) suggest that we “form a rhizome with our viruses, or rather our viruses cause us to form a rhizome with other animals.” All earthly life fundamentally shares one health, as proponents of the multidisciplinary global initiative to tackle zoonosis assert. Spillover diseases expose the vulnerability of species boundaries. Conceived as a taxonomic container of sorts, the concept of species has long established a sense of order (Kirksey 2015). Zoonosis troubles that sense.
Land-use changes, booming human and livestock populations, global travel, biodiversity loss, random mutation, natural selection, and even intentional bioterror are among the many factors that have opened this Pandora’s box. Recent outbreaks such as SARS (probably from horseshoe bats), MERS (from camels), bird flu (from poultry), Ebola (possibly from fruit bats), bubonic plague (from rats and other rodents), and Zika (possibly from rhesus monkeys) demonstrate how human and insect vectors can spread diseases rapidly and over oceans. A warming climate invites mosquitoes, flying syringes loaded with viruses and parasites, to colonize new lands. Airplanes enable infectious agents to incubate in human bodies even as they are transported elsewhere.
In tropical regions such as Madagascar, where I do research, the destruction of biodiverse forests for mines, agriculture, timber, and new construction stirs up unknown viruses and crowds remaining wildlife species into habitat fragments. This, in turn, cultivates more pathogenic environments for humans and other vertebrates. At the boundary of primary habitats and human settlements, viruses that lurk in other animals become active or, to use the language that scientists do, chatty. The term viral chatter denotes a situation in which “a zoonotic pathogen genetically adapts the ability to ‘jump’ into human hosts, but not yet to the degree that it can sustain further transmission in human populations” (Barrett 2010, 84).
I like the double entendre of viral chatter. It points to an internal relationship between rising pathogenesis in ecosystems and rumors about disease outbreaks circulated in the media and in conversation. The association of microbial activity with Internet memes about the cause of outbreaks or the means of transmission is not merely metaphorical. Computer technology is, after all, sustained through the extraction of rare earth and industrial minerals from tropical forest regions.
Disease rumors that go viral confound public health responses. The 2014 Ebola outbreak in West Africa was exacerbated not only by pervasive infrastructural and economic conditions, but also by conspiracies about the nefarious intentions of state actors and foreigners. A widely circulated report about the Zika virus in Brazil blamed biotechnology for the spate of babies born with microcephaly. It claimed that a larvicide, pyriproxifen, manufactured by a Monsanto subsidiary, was the real cause. Another rumor blamed microcephaly on genetically modified mosquitos meant to wipe out the subspecies Aedes aegypti. But the Zika virus itself is most likely to blame for fetal deformities, having mutated over time and space to the point where it has acquired this power.
Erroneous beliefs about causation often reflect people’s fear of technologies that meddle with nature, however mutable the idea of nature might be. Irrational thoughts and actions around zoonotic epidemics are not always seeded in cyberspace or within localized publics, however. During an acute outbreak of the bubonic plague in the rural town of Amparafaravola, Madagascar in November 2014, medical workers initially sought to quash chatter about the plague, denying that it had arrived in town. Some feared gaining a bad reputation for the town as the locus of an epidemic, or getting reprimanded for mishandling public outreach.
The silencing of plague chatter by medical workers no doubt led to the illness of some twenty-seven residents and the preventable deaths of seven; after all, the bubonic plague can be treated with antibiotics if it is caught early enough. The delay in informing people about the means of transmission and about how to handle the highly infectious sick and dead put people at risk of developing and spreading the more lethal pneumonic strain, which has since broken out in other parts of the island.
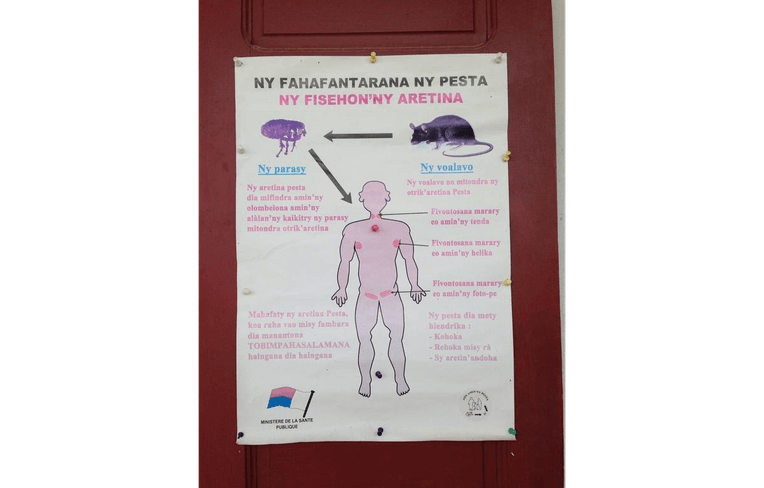
In Madagascar, rats are the main source from which the plague jumps to humans via flea bites. The blood of rats is rich with the plague bacillus, Yersinia pestis. In Amparafaravola, the outbreak peaked during the warm, rainy season, when fleas are abundant and people store mounds of unhulled rice inside or near their homes, unwittingly luring rats into close quarters. Throughout Madagascar, the incidence of the plague has risen over the past few years due to urban overcrowding and poor sanitation, which increases rat-human contact; climate change, which affects the population dynamics of rodents and fleas; and rampant deforestation, which induces rats to migrate into agricultural fields and settlements (McCauley et al. 2015).
Pesticide and antibiotic resistance, difficulties in developing vaccines quickly, and the tangled mix of factors triggering zoonotic outbreaks leave us in thrall to biotechnology, despite pervasive ambivalence about what it means to sculpt evolution. Genetic modification techniques represent a thorny path toward the future, promising to defuse the ticking time bomb of certain problem insects even as they fortify the boundaries of our own posthuman species.
Barrett, Ron. 2010. “Avian Influenza and the Third Epidemiological Transition.” In Plagues and Epidemics: Infected Spaces Past and Present, edited by D. Ann Herring and Alan C. Swedlund, 81–94. Oxford: Berg.
Deleuze, Gilles, and Félix Guattari. 1987. A Thousand Plateaus: Capitalism and Schizophrenia. Translated by Brian Massumi. London: Continuum.
Kirksey, Eben. 2015. “Species: A Praxiographic Study.” Journal of the Royal Anthropological Institute 21, no. 4: 758–80.
McCauley, Douglas J., Daniel J. Salkeld, Hillary S. Young, Rhodes Makundi, Rodolfo Dirzo, Ralph P. Eckerlin, Eric F. Lambin, Lynne Gaffikin, Michele Barry, and Kristofer M. Helgen. 2015. “Effects of Land Use on Plague (Yersinia pestis) Activity in Rodents in Tanzania.” American Journal of Tropical Medicine and Hygiene 92, no. 4: 776–83.